The pharmaceutical industry is – for understandable reasons – subject to very special security and compliance guidelines. This also includes the absolutely flawless and legally compliant documentation of process plants. This is because it serves as the basis for operating permits and, if necessary, for the product release of substances and medicines produced in the plant. For this purpose, the US Food and Drug Administration (FDA) has published a set of rules that has set global standards: Title 21-Food and drugs part 11 (21 eCFR Part 11).
EB’s e-records: compliant by their very nature
Of course, documenting up-to-date and correct plant documentation starts with the contents of the documents. Here AUCOTEC's Engineering Base platform (EB) is optimally positioned "by its very nature" so to speak. Because of its versatile central data model, which can be processed in parallel by all core engineering disciplines, the complete change history of every asset and documentation can be tracked, with all its associations and background information, including who changed what and when. All revisions and version statuses, also including change history, are logged with complete audit trail, and are transparent and traceable in EB– for the entire project.
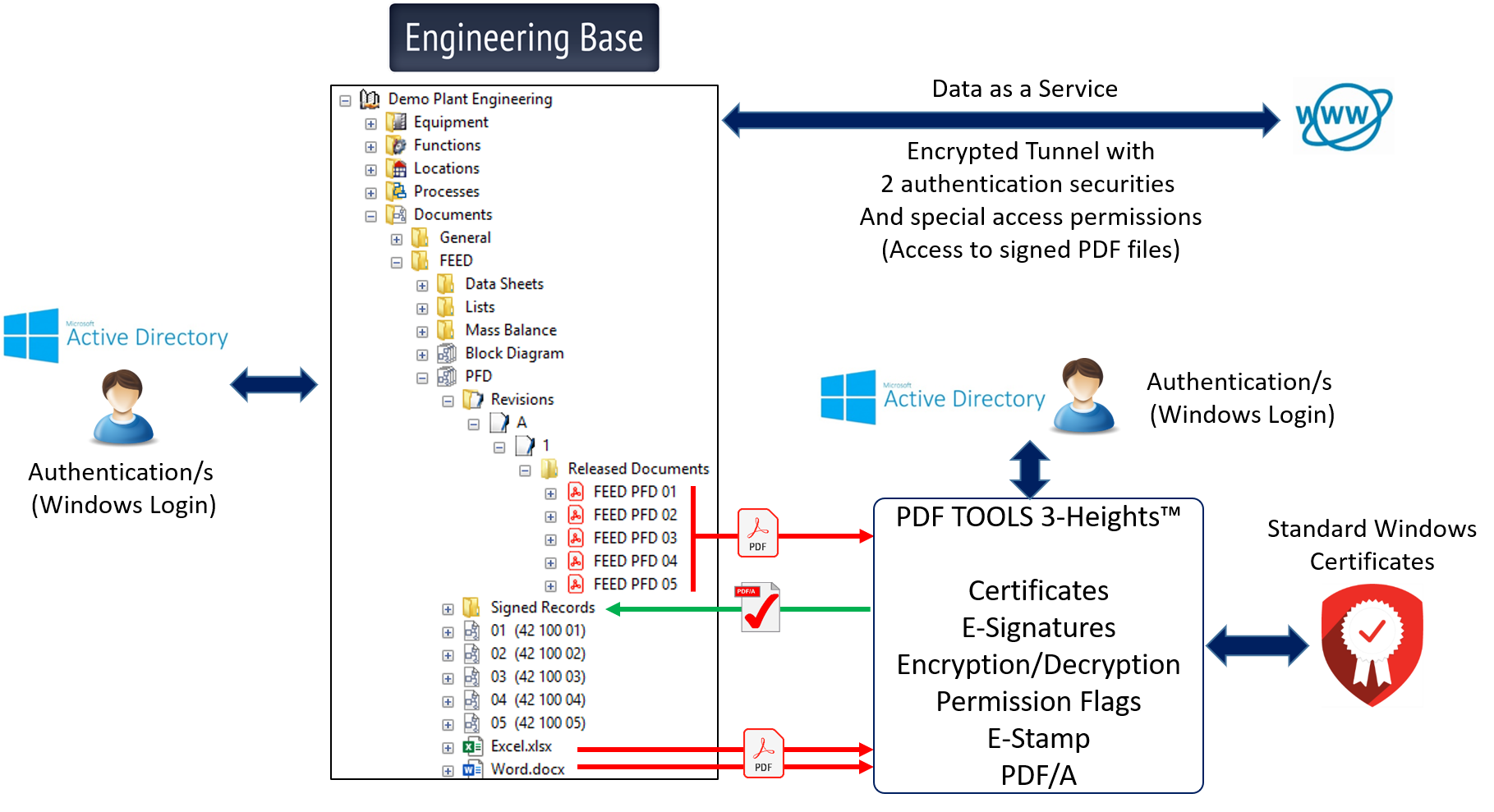
This information can be viewed at any time via web services with encrypted data tunnels and 2-factor authentication. The necessities of having a qualified e-record, which is also often a prerequisite for operating permits, is already covered per se by Engineering Base.
 © AUCOTEC AG
© AUCOTEC AG"I vouch for our quality with my name"
This statement from a well-known German baby food manufacturer is intended to express the highest levels of reliability, responsibility and integrity. The same is true for the signature under plant documents. It is also highly relevant for approval processes in plant engineering and design. But in our digital world, there are no longer any handwritten signatures "signed and sealed". E-signing therefore requires a whole range of security mechanisms to guarantee that the "virtual" electronic signature really belongs to the right real person in charge and can be tracked by authorities at any time.
E-signing by a professional
AUCOTEC also has a solution for EB for this purpose: in addition to the uniqueness of e-signatures, linking between the signature and the certificate for competence to sign, it guarantees with legal certainty a whole range of other required services in this context. In cooperation with the PDF experts of the Swiss PDF Tool AG, based in Zurich, the PDF to PDF/A Converter and PAdES (PDF Advanced Electronic Signature) can be integrated into EB via an API (application programming interface) if required. This allows the desired documents to be transferred to the long-term archiving format PDF/A and digitally signed at the same time.
The basis for this is an Adobe standard technology that supports Windows Active Directory login. All requirements that authorities such as the FDA place on legally compliant, reliable and trustworthy documents are thus covered. For example, time stamps, unique identification codes for a signature, password-protected access configurations and other functions that make a signed document reliable and approvable.


